Argon
What is Argon?
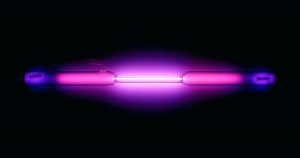
Argon is part of the 18th group of elements which are known as the noble gases. Noble gases are the last group of elements and are known for being highly unreactive. Until relatively recently it was believed these elements were completely inert, meaning they will react with nothing, but some compounds have been found under extreme conditions. Argon is a colorless and odorless gas, but when placed into an electric field, it gives off a beautiful violet glow. Argon has been used historically and in modern times for a variety of things, but in today’s world argon is primarily used as an inert shielding gas for various high temperature industrial processes like welding. Argon is also used in different types of lighting, for example, incandescent and fluorescent.
 Argon in the Periodic Table
Argon in the Periodic Table
Atomic Number: 18
Symbol: Ar
Group: 18
Period: 3
Number of Protons: 18
Number of Electrons: 18
Number of Neutrons: 22
Atomic Radius: 188pm
Atomic Mass: 39.948
Number of Isotopes: 25
Like many elements, the name argon comes from Greek. The Greek translation of the word argon comes out to mean “lazy” or “inactive”. This makes sense as argon is one of the primary inert (unreactive) noble gases found on earth. Argon’s original symbol was “A”, but in 1957 was changed to Ar. Argon was not the only element to have its name changed. The IUPAC was the group who decided upon this.
Properties of Argon
As a colorless and odorless gas, it is rather difficult to portray what argon looks or feels like. Even in its solid and liquid forms, argon is unreactive, non-toxic, and non-flammable. Argon is literally all around us and is one of the most abundant elements that make up our atmosphere. In nature (on the earth at least) atmospheric argon is nearly all in the form of radiogenic argon-40. Radiogenic means to have been produced by radioactive material. This argon comes from the decay of potassium-40 which resides in the earth’s crust. As a noble gas argon itself cannot corrode, but it can be used to prevent corrosion of other materials. Argon has very low thermal conductivity and does not conduct electricity, just like many other gases. Argon is classified as a noble gas. Nobles gases are a unique set of elements as they are inert (unreactive). This is due to their electron configuration. Each noble gas has a complete octet in its outermost shell, meaning that it does not desire to gain or lose any electrons. The entire purpose behind chemical bonding is to reach a stable configuration by sharing electrons. Noble gases do not need this, hence them being unreactive. Obviously, this inertness corresponds with the element being incredibly stable. Noble gases are all extremely stable elements and are always happy to be alone and exist is their monoatomic state. Monoatomic is when there is just one type of atom that exists alone. This is especially common in gases.
Physical Properties of Argon
As a colorless and odorless gas, solid/ liquid argon looks very similar to clear water. In its natural gas form, argon is very difficult to identify. The best way to understand the physical aspects of argon is through the use of an electrical field. When placed inside one, argon glows a very prominent violet color. This has seen some commercial use for different colored lighting.
Melting Point: -184oC
Boiling Point: -185.8oC
Density: 1.784 g/L
Phase at Room Temperature: Gas
“Argon Light” by Cameronmonti is licensed by CC by 2.0
Chemical Properties of Argon
Argon has the same solubility in water as oxygen and is 2.5 times more soluble in water than nitrogen. This gas is non-flammable, colorless, odorless and extremely unreactive. Argon, along with the other noble gases, do not have an electronegativity value. This is due to their lack of reactivity.
Oxidation States: 0
Specific Heat: 0.52 J/g K
Electronegativity: 0
Heat of Fusion: 1.188 kJ/mol
Heat of Vaporization: 6.447 kJ/mol
Electron Configuration: [Ar] or [Ne] 3s2 3p6
Isotopes of Argon
Argon does in fact have a few different isotopes, but, on the earth at least, they are quite skewed in abundance. The most common isotope by far is 40-Ar. This isotope is responsible for atmospheric argon and makes up about 98% of all naturally occurring argon. 36-Ar is the next abundant form and makes up about 0.34% of all argon on earth. The last “common” isotope is 38-Ar which contains about 0.06% of all argon on the planet. 39-Ar is a very interesting isotope due to how it is formed. It does not have any real use but is created by cosmic ray activity in the atmosphere. Cosmic rays are high energy atomic nuclei or other particles that are traveling through space at speeds approaching the speed of light. 40-Ar will go through neutron capture, that is to say it gains another neutron, but this causes a two-neutron emission (or loss of neutrons) leaving the element as 39-Ar. Across the universe the abundance of different isotopes varies greatly. This all depends on the type of environment. In rocky environments, such as the earth, argon produced through decaying potassium will be the most abundant. However, in many gaseous environments, like our sun, argon that is produced by stellar nucleosynthesis will have 36-Ar be the dominant form. Stellar nucleosynthesis is the creation of chemical elements through nuclear fusion reactions in stars. This isotope, 36-Ar, is by far and wide the most abundant form of argon in the universe even though it is not the most common on earth. This makes sense due to the sheer size and abundance of stars in the universe.
Alloys of Argon
Argon does not form alloys.
Occurrence and Abundance of Argon
On the earth, atmospheric argon is by far the most common place it is found. After decaying from 40-potassium, stable 40-Ar takes to the atmosphere. Argon is the third most abundant element in the earth’s atmosphere and makes up 0.934% of every breath we take. This may not sound like much, but when looked at comparatively, argon is about twice as abundant as water vapor, and 25 times more abundant than carbon dioxide. Of the noble gases, argon is also the most prevalent and abundant in the earth’s crust, comprising 0.00015% of all elements. Our major abundance on earth is quite different from the majority of the universe. In stars, 36-Ar is created by stellar nucleosynthesis and is by far the most abundant form outside of our planet and in the universe as a whole. In the universe, argon makes up 0.02% of all elements.
Uses of Argon
Argon is widely used in many industrial and scientific processes. This is for a few main reasons. First, it is an inert gas. This is useful because there is little risk of contamination or byproduct creation when used in the sciences. In industrial terms, this is useful because argon is non-flammable and safe. Second, argon is extremely cheap. Argon is created as a byproduct during cryogenic air separation. Cryogenic air separation is the process in which liquid nitrogen and liquid oxygen are created. These two liquids are very commonly used in industrial processes meaning that they are created in large quantities. Since they are created in bulk, the byproduct, argon, is also created in vast quantities, making it very cheap. Companies use this to their advantage by using the argon in other areas of production. The third reason argon is used is due to its low thermal conductivity. Industrial and scientific communities alike need resources that will not generate more heat as this could damage products.
General Uses of Argon
Argon is used in some high temperature industrial processes. One example is the use of argon in graphite electrical furnaces. An argon atmosphere is used in the furnace to prevent the burning of the graphite. This is effective because argon has a very low thermal conductivity so it is much better at displacing heat when compared to normal atmospheric air. Argon is also used in place of other gases when there is a risk of another gas defecting the material. This is common in arc welding as the presence of oxygen and nitrogen could defect the product. Arc welding is a style in which heat is generated by an electric arc. The same is true for the production of titanium. Another commercial use for argon is in the poultry industry. Argon gas can be used to asphyxiate birds as argon tends to disperse oxygen close to the ground. This is seen as a humane way to slaughter for food and actually has some other added benefits, the most important of which is that by replacing the oxygen levels with argon in the birds the meat will stay fresh longer. This is again due to argon’s inert property. Argon’s ability to extend the shelf life of products is used in other places too. Wine makers use argon to create a border against damaging oxygen and other meat industries sometimes use argon atmospheres in their products for the same reason. In lighting, argon can be an important component in both incandescent and fluorescent lights. Interestingly argon can also be used to extinguish fires. It is not as effective as foam or water but will not damage valuable electronics.
“Argon ice” by Hight3ch.com is licensed under CC by 2.0
Scientific Uses of Argon
Two fascinating uses for argon come from its liquid form. In some neutrino experiments, liquid argon is used as the target. This means that argon is what the experiments themselves are attempting to locate. Liquid argon is also used in dark matter searches. In the lab, argon is used for a few different experiment types. In gas chromatography, argon is used as the carrier gas. Gas chromatography uses a gas to analyze different compounds for things like purity and chemical make-up. Argon is also used as the gas in ICP spectroscopy. That is a method used to analyze and measure chemical samples. Argon also has a few uses in medicine. In some cryosurgery procedures, especially cryoablation, argon plasma beams are used to destroy tumors and other cancerous cells located inside tissue. Argon can also be used to create a blue light laser which is used during surgical procedures to weld arteries, destroy tumors, and even perform eye defect correction surgeries.
Notable Compounds of Argon
Argon has a completed octet of electrons. That is to say its p and s shells are completely filled. This causes it to be inert. The full shell provides an incredibly high level of stability and makes for a very large resistance to bonding. Throughout most of history it has been thought that the noble gases were completely unreactive, and although this is mostly true, some compounds can be synthesized under extreme conditions. The first argon compound was not synthesized until 1975 and it was tungsten pentacarbonyl W(CO)5Ar. Another was made in 2000, argon fluorohydride (HArF), at the University of Helsinki. This compound is stable up to -256oC. There is also argon hydride which is the only known naturally occurring compound. This compound was detected as part of the crab nebula supernova and was the first ever noble gas compound detected in space.
Discovery of Argon
Argon was the first noble gas to be discovered, which is what led the founders to its Greek name of lazy or unreactive. The first person to suspect the presence of argon was Henry Cavendish. In 1785 he, in a series of experiments, thought there must be the presence of an unreactive gas. Despite his inking argon was not truly discovered for over 100 more years. In 1894 Lord Rayleigh and Sir William Ramsay took a sample of clean air and removed the oxygen, carbon dioxide, water, and nitrogen, leaving only argon. They decided to do this after noticing that atmospheric nitrogen was about 5% heavier than nitrogen taken from chemical compounds. This caused them to hypothesize that another element must be present in the atmospheric samples, leading to the discovery of argon.
Interesting Facts about Argon
- Though non-toxic, argon does rather well at displacing oxygen, making it an asphyxiation hazard in confined places
- There are no confirmed room temperature compounds of argon
- Argon’s triple point, the pressure and temp in which all three states are present, is used as a standard in the International Temperature Scale
Argon in the Future
Due to being a cheap, effective, and safe element, argon will continue to be used for the foreseeable future. Argon in industry will stay relevant as long as liquid oxygen and nitrogen do as well, which seems extremely likely. The need for a stable, safe, and inert gas will always be there, and argon performs excellently with filling that role.
 Argon in the Periodic Table
Argon in the Periodic Table