Berkelium
What is Berkelium?
Berkelium is the 97th element of the periodic table and is a radioactive, unstable element that can form metal like sheets. Berkelium is only made in a lab in very small amounts so there are few commercial applications outside of the realm of scientific research.
Berkelium in the Periodic Table
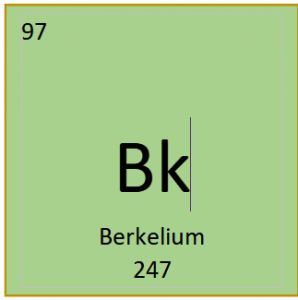
Symbol: Bk
Group: N/a
Period: 7
Number of Protons: 97
Number of Electrons: 97
Number of Neutrons: 150
Atomic Radius: N/a
Atomic mass: 247
Number of Isotopes: zero stable isotopes
Berkelium gets its name from the city of Berkeley, California where the element was discovered in a lab at the University of California, Berkeley. It is categorized as an actinide element in the last row of the periodic table, right below the lanthanide series.
Properties of Berkelium
Berkelium is a radioactive, unstable element that can only be produced in a lab. It can form very small metal sheets, but production of the element is very limited. It can react with oxygen and oxidize slowly when exposed to air. There is no information about the conductive properties of berkelium. It is classified as an actinide element, and also as a transuranium element meaning it is heavier than uranium and therefore farther along in the periodic table. All transuranium elements are unstable and radioactive. Given that the element is radioactive, it is considered toxic to humans and should be handled with great care.
Physical Properties
Berkelium has to be created in a lab so there is little data on its physical properties. For example, there is no known boiling point for the metal. It is, however, denser than common metals like lead and copper, but less dense than gold.
Melting Point: 1323 K (1050°C)
Boiling Point: N/a
Density: 14.78g/cm3
Phase at Room Temperature: solid
Chemical Properties
Just like it’s physical properties, there is little information on the chemical properties of berkelium as well. The most common oxidation state is +3, but it can have a couple other ones as well. There is no information on the heat of fusion or vaporization, and because it’s mostly produced in a pure form in the lab, there is little information on bonding preferences.
Oxidation states: +3
Specific Heat: N/a
Electronegativity: 1.3
Heat of Fusion: N/a
Heat of Vaporization: N/a
Electron Configuration: [Rn]7s25f9
Isotopes
All of the many isotopes or berkelium are considered radioactive and unstable, but the most stable one is 247Bk which has a half-life of 1380 years. This may seem like a long half-life, but there are elements with half-lives that are millions of years long as well. There are about 20 different isotopes that scientists have been able to identify and produce.
Alloys
There are no known alloys of Berkelium, as it is an unstable, man-made element with no known commercial uses.
Occurrence and Abundance of Berkelium
Berkelium does not have any occurrence in nature and therefore does not have any abundance. The element can only be produced in a lab setting and is generally only made in very small amounts. It is most commonly produced by bombarding actinides like uranium or plutonium with neutrons in a nuclear reactor.
Uses of Berkelium
There is no known general commercial use for berkelium. It is strictly used in scientific research, and even within the research field, is really only used as a starting point in the production of even heavier, radioactive elements, such as lawrencium.
Notable Compounds
There are a couple of berkelium compounds that have been formed, but none of them have any commercial value. There are a couple different oxides of berkelium such as berkelium dioxide (BkO2) and berkelium oxide (BkO). There is also berkelium trifluoride (BkF3), berkelium trichloride (BkCl3) and berkelium triiodide (BkI3).
Discovery of Berkelium
Berkelium was first discovered, or produced, in 1949 by a team of researchers at the University of California, Berkeley. This team was able to discover 11 transuranium elements between 1940 and 1961 because of a particle accelerator that was new to campus. To discover berkelium, the team fired alpha particles, or helium atoms with no electrons, at americium atoms in this particle accelerator.
Interesting Facts about Berkelium
- It took 9 years to finally produce enough berkelium that it could be seen by the naked eye.
- The first compound of berkelium, which was berkelium oxide, was first produced in 1962.
- One of the scientists who discovered berkelium, Glen Seaborg, also discovered 9 other elements.
- Berkelium was the fifth element in the periodic table after the last naturally occurring element, uranium, to be artificially synthesized.
- Most experiments to determine physical and chemical properties of berkelium are done on the 249Bk isotope.
Berkelium in the future
Most of the research focused on berkelium is fairly limited, especially because there are still very basic things like physical and chemical properties that are unknown about the element. Radiation laboratories are working on being able to measure more of these attributes, but given there is no known commercial viability for berkelium, there is little focus on such projects.