Astatine
What is Astatine?
Astatine is the 85th element on the period table and has frequent controversy surrounding its classification. It is denoted by the symbol At. The issues arise from the extreme radioactivity of the element. Some scientists classify astatine as a metalloid, others and a metal, and others yet as a non-metal. The reality of the situation is that a macroscopic sample of astatine cannot be obtained since any larger sample would be immediately vaporized by the heat of its own radioactivity. Obviously due to this we have no real understanding of the elements natural appearance and astatine is only found in the decay of other elements. Astatine has begun to see some use in medicine but research on its usefulness is ongoing as the extreme volatility of the element makes it difficult to work with.
 Astatine in the Periodic Table
Astatine in the Periodic Table
Atomic Number: 85
Symbol: At
Group: 17
Period: 6
Number of Protons: 85
Number of Electrons: 85
Number of Neutrons: 125
Atomic Radius: 127pm
Atomic Mass: 210u
Number of Isotopes: 39
Astatine has a long history full of unknowns; including its name. During the time of astatine’s discovery non-naturally occurring elements were seen as less legitimate than naturally occurring ones. So, the chemists surrounding the discovery attempted to find it in nature before naming it and claiming the discovery. Once it was realized that the element only occurred during the decay of others, it was decided that the name astatine was appropriate. The name comes from the Greek astatos, meaning “unstable”. Nearly all of the known, or rather hypothesized, information about the properties of astatine come from the study of its neighboring elements, especially iodine.
Properties of Astatine
Not much is known about astatine due to its high radioactivity and very short half-lives. In nature, it is only found as a product of decay from other elements, never as a standalone element. Astatine is very dangerous and must be handled with extreme care.
Physical Properties
As mentioned above it would be impossible at this time to assemble a macroscopic sample of astatine. Therefore, the classification, appearance, and most other physical properties are unknown, or taken from data extrapolation. For example, halogens follow a trend in which they become darker as they increase in weight. Fluorine is nearly colorless, chlorine has a yellow-green hue, bromine is red-brown, and iodine is a deep violet. If we extend this trend it can be hypothesized that the appearance of astatine is most likely black or close to black. Below is an image of what astatine’s proposed appearance would be.
Melting Point: 302oC
Boiling Point: 337oC
Density: ~7g/cm3
Phase at Room Temperature: solid
“Astatine” by chemistryscore.com is licensed under CC BY 2.0
Chemical Properties of Astatine
The study of the chemical properties of astatine has been forever hindered by the extremely small scale they must be performed on. This type of experimentation is useful but can generally not be considered fully conclusive as experiments conducted on that scale are prone to many inconsistencies. The astatine must be diluted to absurd levels to even have a chance for experimentation, around 10-10molL-1. How astatine interacts with many elements is simply unknown and it is generally extremely inert. Astatine has the same electronegativity as hydrogen and interestingly in hydrogen astatide the negative charge is predicted to be on the hydrogen atom.
Oxidation States: -1, +1, +3, +5, +7
Specific Heat: unknown
Electronegativity: Pauling Scale 2.2
Heat of Fusion: unknown
Heat of Vaporization: unknown
Electron Configuration: Xe 4f14 5d10 6s2 6p5
Isotopes
Astatine has 39 known isotopes. The isotopes have mass numbers from 191-229 and models created on astatine suggest that there are 37 more isotopes yet to be discovered. None of these isotopes are stable or long-lived and it is highly unlikely that there is such an isotope. Isotopes of astatine generally follow an alpha decay trend for the lighter ones and beta decay for the heavier isotopes. The most stable isotope of astatine is 210At which has a half-life of only 8.1 hours. Astatine only has 5 isotopes that have a half-life longer than one hour and most are much shorter. The shortest-lived isotope is 213At which has a half-life of only 125 nanoseconds. Astatine 219 is the longest-lived naturally occurring isotope and has a half-life of 56 seconds.
Alloys of Astatine
There are no known astatine alloys.
Occurrence and Abundance of Astatine
Astatine is the rarest naturally occurring element on the earth. The total amount of astatine in the earth’s crust is thought to be less than one gram at any given time. There is no astatine on the earth today that was here during the earth’s formation, it has all long since decayed and disappeared. There are four naturally occurring isotopes, astatine 215, 217, 218, and 219, and all of these are only produced by the decay of uranium and thorium ore. Astatine is also sparingly produced from the decay of neptunium237. Astatine is produced synthetically in most of its forms, which is actually how it was first discovered. The abundance of astatine is so negligible in the earth’s crust and in the atmosphere as a whole that no value can be determined.
General Uses of Astatine
Astatine has no general or commercial uses at this time.
Scientific Uses of Astatine
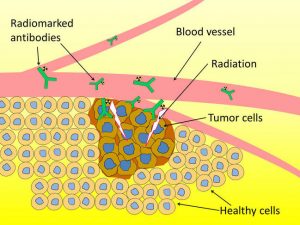
Astatine has begun to see use in some facets of medicine. It is currently being research for use as an alpha particle in nuclear medicine. This development could take some time as the voltaic nature of the element make progress difficult. Astatine is also very toxic and dangerous to work with, which inhibits studies.
“Astatine-211-Anti-body Combination” by F. Guerard is licensed under CC BY 2.0
Discovery of Astatine
When the periodic table was first published in 1869 by Dmitri Mendeleev a block was left under iodine as it was known that an element should be placed there but had yet to be discovered. Before its official recognition astatine was known as “eka-iodine” much as many other yet to be discovered elements were known as “eka- X”. The first claim to discover the element was made in 1931 by Fred Allison. He named the element alabamine and this was accepted for a few years. In 1934 H.G MacPherson disproved Allison’s claims. Another claim was made in 1937 but it was shown that the proposed element did not correspond to the known properties of eka-iodine. Similar claims were again made in 1936, 1940, and 1942. However, none of these were ever validated fully. In late 1940 Dale Corson, Kenneth Roth Mackenzie, and Emilio Segre isolated the element at UC Berkley. They opted to not search for the element in nature and instead bombard bismuth-209 with alpha particles. This caused neutron emission and after the loss of two neutrons astatine-211 was left. The scientists did not name their element however. This was because the validity of an “invisible quantity” of a new element that was produced in a lab had very little traction at the time. During this time in chemistry synthetically derived elements garnered little respect, as did elements that were completely unstable. In 1943 astatine was found naturally as a product in the decay of uranium. It wasn’t until 1946 that Friedrich Paneth called to finally recognize synthetic elements onto the periodic table. In 1947 astatine was finally placed onto the table.
Interesting Facts about Astatine
- Astatine can become easily concentrated in the thyroid gland if exposed
- World War 2 delayed astatine studies by nearly a decade
- Some naturally occurring isotopes are effectively extinct in nature as their precursors have all disappeared
Astatine in the Future
Astatine has seen some promise in the field of medicine. It is very effective in preliminary cancer treatments however and it has been noted that if develop proves to be useful for large quantities of people that the amount of astatine available could become an issue.
 Astatine in the Periodic Table
Astatine in the Periodic Table