Bromine
What is Bromine?
Bromine is a non-metal in group 17, period 4 of the periodic table. Pure bromine exists as a brown liquid at standard temperature and pressure. Bromine is used in chemical reactions to make flame retardants, pesticides, medications, and pH sensitive dyes.
Bromine’s Place in the Periodic Table
Bromine is a halogen like all the elements located in group 17. Halogens can exist as gases, liquids, or solids at room temperature. Because bromine is in period 4, in the middle of the halogen group, it has properties that are intermediate to the rest of the halogens. It is the only non-metal on the periodic table that is liquid at room temperature. The name bromine refers to the Greek word, bromos, for stench or bad smell.
- Atomic number: 35
- Atomic Radius: 120 picometers
- Atomic mass: 79.901
- Symbol: Br
- Group: 17
- Period: 4
- Number of Protons: 35
- Number of Electrons: 35
- Number of Neutrons: 44 (51% abundance), 46 (49% abundance)
- Number of Isotopes: 2 naturally occurring
Properties of Bromine

Physical Properties
Bromine is solid at temperatures below −7.2 °C, which is about the temperature inside of your freezer at home. Bromine is liquid up to 58.8 °C. That is about as hot as some of the hottest deserts on Earth, like Death Valley, California. That also makes it the only non-metal that is liquid at room temperature. Bromine (Br2) is about 3-times the density of water. That is about the same density of a solid Aluminum.
- Melting Point: -2 °C.
- Boiling Point: 8 °C.
- Density of Liquid Bromine: 1028 g cm-3
- Phase at Room Temperature: liquid
Chemical Properties
Pure bromine exists as Br2 and exists stably at room temperature if stored in an air-tight container. It has seven valence electrons (Fig3), so is only missing one electron to achieve the stability of 8 valence electrons, which is what makes it very reactive. Bromine can exist at multiple oxidation states, so it can form a wide variety of compounds. It is a strong oxidizing agent and forms compounds with metals (FeBr3), non-metals (HBr), and even other elements in group 17 elements (BrF, BrCl, Br2).
- Oxidation states: −1, +1, +3, +4, +5, +7
- Specific Heat: .473 j g-1 K-1
- Electronegativity: 2.96 (Pauling scale)
- Heat of Fusion: 571 kj mol-1
- Heat of Vaporization: 96 kj mol-1
- Electron Configuration: [Ar] 3d10 4s2 4p5
Isotopes
Bromine and chlorine are the only halogens that have more than one natural isotope. Two naturally occurring isotopes of Bromine exist in about equal proportions. Br-79 is about 51% of all bromine, and Br-81 is the rest. That’s why the atomic mass of bromine is about halfway between 79 and 81. These isotopes are stable. All other bromine isotopes that are produced in laboratories are radioactive. They range from Br-66 to Br-97, with a half-life from nanoseconds to several hours.
Alloys and Allotropes
Bromine is not used in the production of alloys.
Compounds of Bromine
Given the high reactivity of bromine, nearly all elements on the periodic table form compounds with bromine. A few exceptions exist for elements that are not reactive (i.e. inert) like the noble gases. The simplest bromine compound is hydrogen bromide (HBr). HBr exists as a gas and when dissolved in water it becomes a strong acid because the bond between hydrogen and bromine is weak, allowing hydrogen ions to be easily released into solution.
Bromides of transition metals can be produced with Br2. This usually forms a covalent bond. Reaction of bromine with non-transition metals, like those in group 1 and group 2, usually form ionic bonds. The formation of bonds between carbon and bromine results in the formation of a broad family of compounds known as organobromines. They are the most common organic compounds that contain halogens in nature (e.g. organofluorines and organochlorines). For example, the ocean releases a million tons of bromoform (CHBr3) and 56000 tons of bromomethane (CH3Br). A million tons is about the weight of water from 250 Olympic swimming pools.
Interesting Facts about Bromine
- Tyrian purple is a dye that was used by ancient civilizations. It was harvested from mussels, a shellfish commonly found in seafood. It was discovered in 1909 that this dye contained bromine.
- We all wish chemical weapons didn’t exist. But they do and the chemical weapons used in World War I contained bromine.
- Be very careful in the laboratory with bromine. It has a bad reputation for causing severe burns, and the vapors can cause chemical pneumonia.
Occurrence and Abundance of Bromine
Of all of the elements on Earth, bromine is the 44th most abundant. It is less abundant than other halogens, such as fluorine and chlorine. It exists as ionic salts in Earth’s crust at about 2.5 parts per million. That’s a ratio of about 2 people in the city of Dallas, Texas. Because it exists as salts that are soluble in water, bromine tends to occur at high concentrations in salt lakes and mineral waters. For that reason, pure bromine is most easily extracted from those sources. The main sources of bromine are the United States and Israel.
Uses of Bromine
Most Notable Uses in General
Organic bromine compounds are commonly used as pesticides for agriculture. Certain organic bromine compounds also have flame retardant properties, and were once used commonly in fire extinguishers. However, it was later discovered that these compounds were contributing to depletion of the Earth’s ozone layer. Chemical solutions used to develop photographic film contain bromine. In the past, gasoline with lead added (i.e. diesel gasoline) were used as a fuel in cars. So, to prevent lead accumulation in the engine, compounds with bromine were added to gasoline. Diesel gas is not used in most cars these days, so there is less need for bromine additives.
Most Notable Uses in Science
Bromine used to be commonly used in medicines. Bromine compounds have a sedative effect, and have been used to treat epilepsy. However, these compounds were toxic, so were eventually replaced with safer compounds. The food and drug administration no longer approves its use as a medicine. In the chemistry laboratory, bromine compounds are also used as pH indicators. Bromophenol blue for example, is yellow at pH 3.0 and blue at pH 4.6. Ethidium bromide is an organic compound use by scientists to visualize DNA. It gets stuck in the DNA molecule, so when DNA is exposed to ultraviolet light, it fluoresces (Fig. 4).
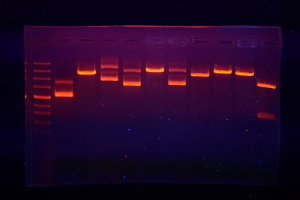
Fig. 4
Discovery of Bromine
Bromine was discovered by two different chemists independently. The first was Carl Jacob Löwig in Germany. He isolated bromine from mineral water produced by a local spring. He concentrated the water and extracted bromine. Antoine Balard, from France, was the second chemist to discover bromine. He knew that other halogens, like Iodine, were present in seaweed. Ashes are salts left over after organic matter is burned. So he used seaweed ashes to create a salt solution, which he then distilled to produce a brown liquid that had properties similar to other halogens, like chlorine and iodine.
Bromine in the Future
Zinc bromine batteries are currently being developed with advantages to traditional lead acid batteries and lithium ion batteries. More energy can be stored in zinc bromine batteries, and they can be discharged completely and recharged completely on a daily basis. The reason is that they don’t have a life-span like lithium ion and lead acid batteries. There is even a company in Australia that is trying to develop a flexible zinc bromine battery, making it possible to fit into inconvenient shapes.