Tin
What is Tin?
Tin is a post-transition metal that is soft, malleable, ductile, and silvery white. It has the largest number of stable isotopes of any element. It was first used during the bronze age (3000 BC) and may be most widely known for its use in “tin cans.” Additional applications include soldering, LCD displays, batteries, anti-cavity treatments, and biocides.
Tin’s Place in the Periodic Table
Tin is a post-transition metal in period 5 and group 14. Post-transition metals are located between transition metals (Cu, Ag, Hg, Cn) to the left on the periodic table and metalloids (B, Si, As, Ge, Sb, Te) to the right. It has a chemical stability similar to lead and germanium. Some forms of tin are paramagnetic, which means that they are weakly attracted to magnets. Alternatively, some forms are diamagnetic, which means that they are repelled by magnets.
- Atomic number: 50
- Atomic Radius: 140 picometers
- Atomic mass: 118.71
- Symbol: Sn
- Group: 14
- Period: 5
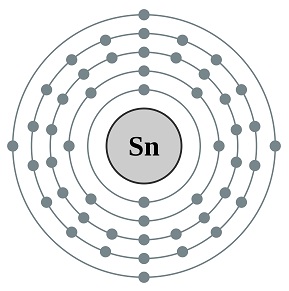
- Number of Protons: 50
- Number of Electrons: 50
- Number of Neutrons: ~68
- Number of Isotopes: 10 natural isotopes
Properties of Tin
Tin has the lowest melting temperature of any group 14 element. There are two forms of tin that have distinct crystal structures, or arrangement of atoms as a solid. a-Tin is known as gray tin. It is only stable at low temperatures (less than 13.2°C). It is brittle and does not have metallic properties because the atoms are bonded covalently. These properties limit its applications as a metal. b-tin is known as white tin. It is stable at room temperature and above. It has metallic properties and is malleable. Tin is stable in water but reacts with strong acids and bases. Tin conducts heat and electricity as well as iron and platinum but about 5-times less than the best conductors, copper and silver. Tin has multiple stable oxidation states, but the main ones are +2 and +4. It has no known biological role and can be toxic in certain chemical forms.
Physical Properties
Tin is solid at room temperature and has a density similar to zinc and iron. It melts at 291.93°C. At that temperature, paper would spontaneously combust.
- Melting Point: 291.93°C.
- Boiling Point: 2602°C.
- Density of Solid Tin: 26 g cm-3 (white tin), 5.77 g cm-3 (gray tin)
- Phase at Room Temperature: solid
Chemical Properties
Tin has 4 valence electrons. There are 2 in 5s orbital and 2 in the 5p orbital. The most common oxidation states are +2 and +4. This is due to electrostatic attraction between the outermost shell and the nucleus. The more electrons in the outer shell, the greater the electrostatic force. So, the +4 oxidations state eliminates the s and p orbitals, increasing the number of the electrons in the outer orbital to 10.
- Oxidation states: -4, -3, -2, -1, +1, +2, +3, +4
- Specific Heat: .21 J g-1K-1
- Electronegativity: 1.96 (Pauling scale)
- Heat of Fusion: 03 kj mol-1
- Heat of Vaporization: 296.1 kj mol-1
- Electron Configuration: [Kr] 4d105s2 5p2
Isotopes
Tin has 10 stable isotopes. That is the largest number of any element. The stable isotopes range from tin-112 to tin-126. The abundance of tin-116, -118, and -120 makes up about 70% of all isotopes. It also occurs in 29 unstable isotopes, ranging from tin-99 to tin-137. Tin-126 has a half-life of 230,000 years, and the rest have half-lives of less than 1 year. Radioactive tin isotope, tin-117, is used as a radioisotope in the treatment of cancer.
Alloys and Allotropes
About half of all tin-containing alloys are used for solders. Solders have low melting temperatures, and so are used to join electrical circuits and metal pipes. Tin bonds easily to other metals, and so it’s used as an anti-corrosive coating on lead, zinc, and steel. Pewter is an alloy dominated by tin. Pewter was widely used as a decorative metal in ancient Egypt. It was used to make bowls, mugs, and plates before the invention of porcelain. It was also used as the base metal for coating with more precious metals, such as silver. Tin is also 12% of the common alloy, bronze.
Compounds of Tin
Tin oxide is amphoteric. Amphoteric is used to describe compounds that can dissolve in both acidic and basic solutions. Tin sulfide is known as mosaic gold. It has a yellow, crystalline appearance, and so can be added to paintings to give a unique reflective property. Diethyltin diiodide ((C2H5)2SnI2) is used as a biocidal agent to prevent biofilm formation. Biofilms are layers of living microorganisms on surfaces.
Interesting Facts about Tin
- Do youkow about those gold Oscar sculptures that are awarded to great actors? Well, they are not made entirely of gold. They are gold-plated metal that is composed of 92 percent tin.
- When bent at room temperature, pure tin emits a screeching sound known as the “tin cry.”
- Tin was used to make musical organ pipes. When temperatures dropped below 13 °C, the a-tin converted to b-tin, which formed a powder on the surface of the pipes. When this was first observed in European cathedrals, it was said that the devil was responsible for the change in the organ pipes.
Occurrence and Abundance of Tin

Uses of Tin
Most Notable Uses in General
Tin has many common uses. Historically, it was used in the fabrications of bronze materials as far back as 3000 BC. Today, we most commonly hear of “tin cans,” used to store food for long periods of time at room temperature. In fact, tin cans are not made of tin but are tin-plated. Tin cans are made of steel that is covered in tin. Tin does not react with water and forms a protective oxidized layer that protects the steel from corrosion. In terms of tin production, it is mostly used to make solders. Solders are metal alloys with low melting temperatures that can be used to join electrical circuits and metal pipes. Tin oxide is similar to indium oxide in that they can both form thin, transparent layers on glass. These layers can conduct electricity and are used commonly to make LCD television screens. Tin is also used as negative electrodes found in lithium ion batteries.
Most Notable Uses in Science
Tin can also be used to treat dental cavities. We have all heard of fluoride supplements in toothpastes and tap water. Sodium fluoride is most commonly used. However, researchers have found that tin fluoride is more stable.
Discovery of Tin
Tin was first used in Mesopotamia, where present-day Iraq is found. So, it is not known who discovered tin originally. The word, tin, is derived from Germanic languages, such as German (zinn), Swedish (tenn), and Dutch (tin). The symbol for tin, Sm, is derived from the Latin name, stannum, which was used to describe alloys of silver and lead.
Tin in the Future
Researchers have recently invested in a tin material that is only one atom thick and can conduct electricity. It is called stanene. Scientists are excited about this material because it could be the only material that conduct electricity at 100% efficiency at room temperature. This is significant because this property is usually reserved for superconductors, which are only 100% efficient at very low temperatures. Stanene has the potential to be used in electronics that consume less energy and produce less heat.