Promethium
What is Promethium?
Promethium is a lanthanide metal. It has a lustrous metallic appearance. It is radioactive and can emit X-rays. It is used in the production of luminous paints, atomic batteries, and calibrations of measurements of thickness.
Promethium’s Place in the Periodic Table
Promethium is a lanthanide metal in period 6. Lanthanides have atomic numbers of 57 to 71, and are found in period 6 and 7. They are named after the element lanthanum – the firth in this series of elements. They all have a similar atomic radius and high electrical resistance. They are also paramagnetic, which means that they are weakly attracted to magnets, but do not retain any magnetic properties when removed from the magnet. Promethium exists together with uranium ores in nature.
- Atomic number: 61
- Atomic Radius: 183 picometers
- Atomic mass: 145
- Symbol: Pm
- Group: N/A
- Period: 6
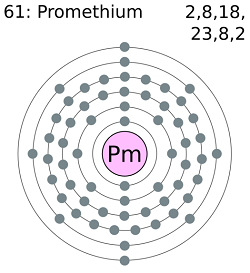
- Number of Protons: 61
- Number of Electrons: 61
- Number of Neutrons: ~86
- Number of Isotopes: 2 natural isotopes
Properties of Promethium
Physical Properties
Promethium is solid at room temperature and has a density similar to zinc and iron. It melts at 1042°C, which is close to the temperature of lava and the melting points of silver and brass. It has a boiling point close to that of lead at 3000°C.
- Melting Point: 1042°C.
- Boiling Point: 3000°C.
- Density of Solid Promethium: 26 g cm-3.
- Phase at Room Temperature: solid
Chemical Properties
- Oxidation states: +3
- Specific Heat: .18 J g-1K-1
- Electronegativity: 1.13 (Pauling scale)
- Heat of Fusion: 13 kj mol-1
- Heat of Vaporization: 289 kj mol-1
- Electron Configuration: [Xe] 4f56s2
Isotopes
All of the isotopes of promethium are radioactive and have relatively short half-lives. Because radioactive decay of promethium produces neodymium, promethium only exists in very small amounts. Trace amounts of promethium-145 and promethium-147 exist in nature. Promethium-145 has a half-life of about 17 years, and promethium-147 has a half-life of about 2 years and 7 months. Promethium-146 can be synthesized in laboratories and has a half-life of 5 years and 6 months.
Alloys and Allotropes
Promethium is not used to form metal alloys.
Compounds of Promethium
Compounds of promethium are usually red or pink in color. PmCl3 can be produced when promethium is dissolved in hydrochloric acid. This compound is used to produce luminous paint that contains a phosphor. Phosphors absorb energy from radiation and emit light. By combining the phosphor with promethium, it is possible to produce light-emitting paint that is stable for a few years. Pm(OH)3 can be produced by adding ammonia to an acidic solution of promethium. Pm(NO3)3 is produced when promethium is dissolved in nitric acid. These compounds can be used for the applications mentioned in Uses of Prometium. PmF3 + 3 Li → Pm + 3 LiF was the reaction used to produce pure promethium under a vacuum.
Interesting Facts about Promethium
- Promethium was identified in the spectrum of light emitted from a star in the Andromeda constellation about 157 light years from Earth. Light years are the time it takes to travel a distance at the speed of light (299,792,458 m s-1 or 186,000 miles per second).
- Promethium was discovered in the Oak Ridge National Laboratory in Knoxville, Tennessee.
- Promethium was the last of the rare Earth metals to be discovered.
Occurrence and Abundance of Promethium
Promethium is very rare and can be found naturally in the mineral uraninite. There are only about 500 grams (about 1 pound) of natural promethium on Earth at any given time. It is produced from the radioactive decay of europium-151 and uranium. It is so rare that there is no accurate calculation for its abundance in the universe. It can be produced in laboratories by bombarding uranium-235 and neodymium-147 with neutrons. Russia is the only country that produces promethium on a large scale.
Uses of Promethium
Most Notable Uses in General
Promethium has no large-scale uses because of its short half-life and rarity.
Most Notable Uses in Science
Promethium compounds are used for some research applications. The radiation that is emitted by promethium is not very strong, so it is safer to use than other radiation sources. For example, atomic batteries use promethium as a radiation source. Radiation emitted by promethium can produce electricity when absorbed by semi-conductors. Additionally, weak radiation cannot travel through dense or thick material. The radiation emitted by promethium can be used to precisely measure the thickness of materials because the thicker the material the less radiation can penetrate. The amount of radiation detected on the side of material opposite the radiation source is proportional to the thickness of the material.
Discovery of Promethium
Promethium was discovered and characterized in 1945 by Jacob A. Marinsky, Lawrence E. Glendenin and Charles D. Coryell. They were able to purify promethium from the decay of uranium fuel. The name is derived from the name Prometheus. In Greek mythology, Prometheus is a Titan who stole fire from Mt. Olympus and gave it to humans.
Promethium in the Future
Scientists continue to study promethium as a source of radiation that can be used to create atomic batteries that can power devices in space and medical implants, such as pacemakers. Pacemakers emit electric shocks that maintain a constant heart rate.
